
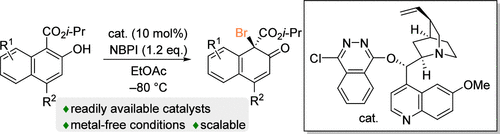
Asymmetric Brominative Dearomatization of 2-Naphthols Using a Cinchona Alkaloid-Based Organocatalyst
Kouhei Omae, Yoshihiro Miyake, and Mio Shimogaki
J. Org. Chem. 2024, 89, 4232−4236.
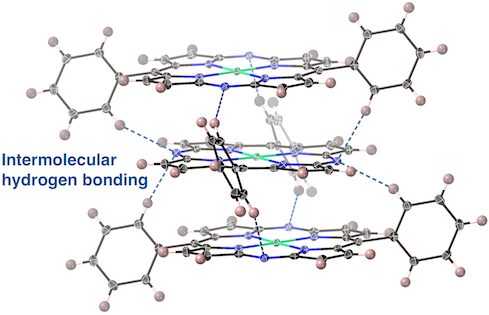
One-dimensional Stacking Array of 10,20-Diphenyl-5,15-diazaporphyrin Metal Complexes
S. Mori, T. Sakurai, T. Nishimura, N. Fukui, Y. Miyake, H. Shinokubo
J. Porphyrins Phthalocyanines 2023, 27, 1035-1041.
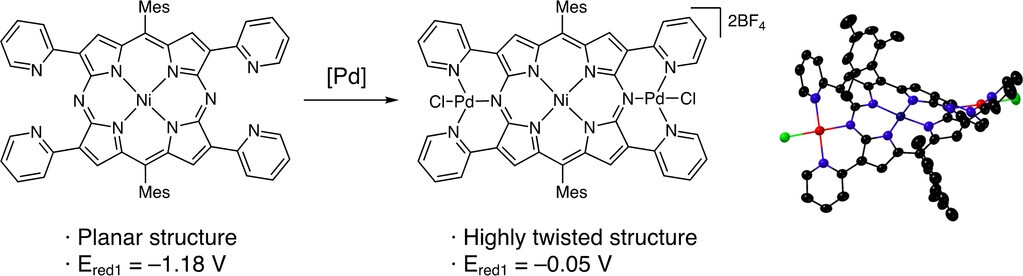
Easily Switchable 18π-, 19π-, and 20π-Conjugation of Diazaporphyrin Double-Pincer Bispalladium Complexes
T. Sakurai, Y. Hiraoka, H. Tanaka, Y. Miyake, N. Fukui, H. Shinokubo
Angew. Chem. Int. Ed. 2023, 62, e202300437.
Stepwise N-Methylation of Ruthenium and Cobalt 5,15-Diazaporphyrins: Post-Functionalization of Porphyrinoid Catalysts
M. Nishijo, S. Mori, T. Nishimura, H. Shinokubo, Y. Miyake
Chem. Asian J. 2022, 17, e202200305.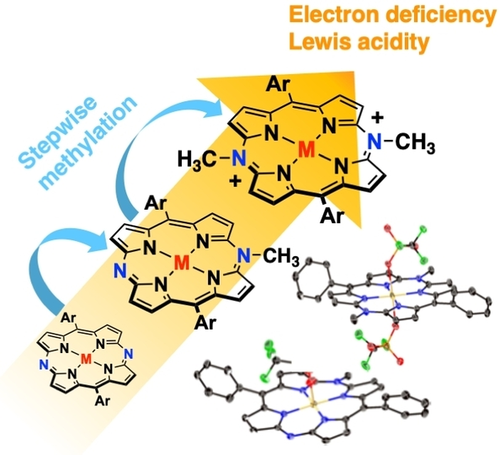
Protonation-Induced Antiaromaticity in Octaaza[8]circulenes: Cyclooctatetraene Scaffolds Constrained with Four Amidine Moieties
S. Akahori, A. Kaga, J. Kim, H. Yorimitsu, D. Kim, H. Shinokubo, Y. Miyake
Chem. Asian J. 2022, 17, e202200244.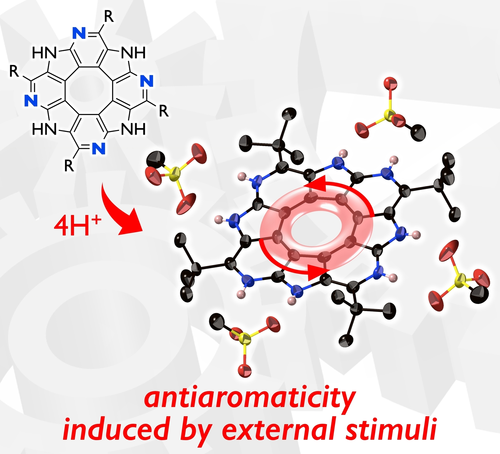
Manganese(III) 5,15-Diazaporphyrins: Synthesis, Properties, and Catalytic Use for Benzylic C–H Fluorination
S. Mori, T. Nishimura, H. Shinokubo, Y. Miyake
J. Porphyrins Phthalocyanines 2021, 25, 991-996.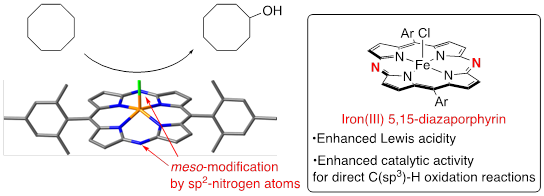
Synthesis and Characterization of 16π Antiaromatic 2,7-Dihydrodiazapyrenes: Antiaromatic Polycyclic Hydrocarbons with Embedded Nitrogen
T. Nakazato, H. Takekoshi, T. Sakurai, H. Shinokubo, Y. Miyake
Angew. Chem. Int. Ed. 2021, 60, 13877-13881.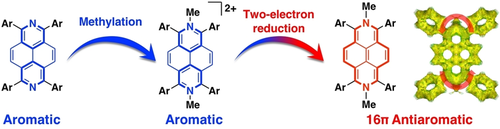
Iron Hexamesityl-5,15-Diazaporphyrin: Synthesis, Structure and Catalytic Use for Direct Oxidation of sp3 C–H Bonds
T. Nishimura, T. Sakurai, H. Shinokubo, Y. Miyake
Dalton Trans. 2021, 50, 6343-6348.
Synthesis of Tetrasilatetrathia[8]circulenes through C–I and C–H Silylation
S. Akahori, T. Fujihara, Y. Tsuji, H. Shinokubo, Y. Miyake
Synthesis 2021, 53, 2995-3000.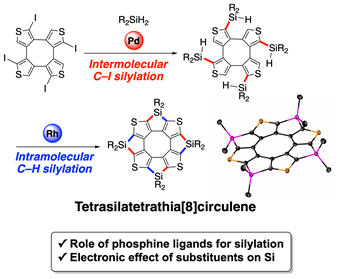
Quadruply BN-Fused Tetrathia[8]circulenes with Flexible Frameworks: Synthesis, Structures and Properties
S. Akahori, T. Sasamori, H. Shinokubo, Y. Miyake
Chem. Eur. J. 2021, 27, 8178-8184.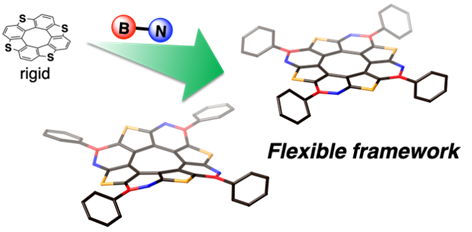
Peripherally Arylated 2,8-Diazaperylenes from Anthracene Diimide: Synthesis and Oxidative Annulation
T. Sakurai, T. Nakazato, H. Shinokubo, Y. Miyake
Org. Lett. 2021, 23, 2099-2103.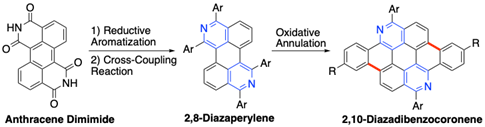
Cationic Nickel(II) Pyridinophane Complexes: Synthesis, Structures, and Catalytic activities for C–H oxidation
T. Nishimura, Y. Ando, H. Shinokubo, Y. Miyake
Chem. Lett. 2021, 50, 1049-1052.
Complexation of 2,7-Diazapyrene with Boron for Structural and Electronic Tuning
T. Nakazato, H. Shinokubo Y. Miyake
Chem. Commun. 2021, 57, 327-330.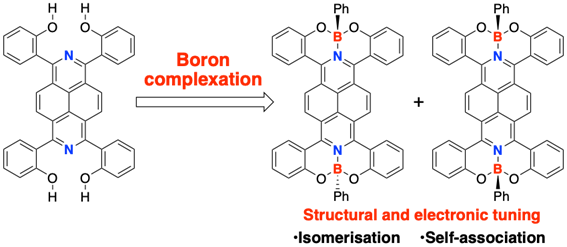
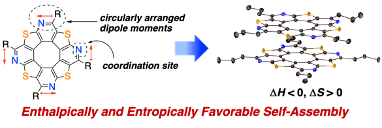
Enthalpically and Entropically Favorable Self-Assembly: Synthesis of C4h-Symmetric Tetraazatetrathia[8]circulenes by Regioselective Introduction of Pyridine Rings
S. Akahori, T. Sasamori, H. Shinokubo, Y. Miyake
Chem. Eur. J. 2021, 27, 5675-5682.
Iron(III) 5,15-Diazaporphyrin Catalysts for the Direct Oxidation of C(sp3)–H Bonds
T. Nishimura, T. Ikeue, O. Shoji, H. Shinokubo, Y. Miyake
Inorg. Chem. 2020, 59, 15751-15756.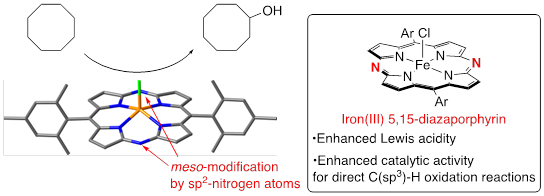
Aggregation-Induced Emission in Tetrathia[8]circulene Octaoxides via Restriction of the Dynamic Motion of their Negatively Curved π-Frameworks
H. Murase, Y. Nagata, S. Akahori, H. Shinokubo, Y. Miyake
Chem. Asian J. 2020, 15, 3873-3877.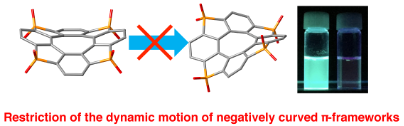
Synthesis and Crystal Packing Structures of 2,7-Diazapyrenes with Various Alkyl Groups at 1,3,6,8-Positions
T. Nakazato, W. Matsuda, T. Sakurai, S. Seki, H. Shinokubo, Y. Miyake
Chem. Lett. 2020, 49, 465-468.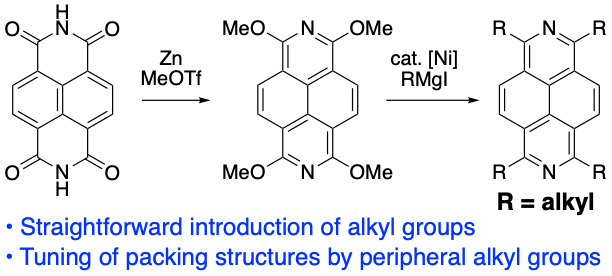
Site‐Selective N‐Methylation of 5,15‐Diazaporphyrins: Reactive Cationic Porphyrinoids that Provide Isoporphyrin Analogues
W. X. Chia, M. Nishijo, S. Kang, J. Oh, T. Nishimura, H. Omori, J. Longevial, Y. Miyake, D. Kim, H. Shinokubo
Chem. Eur. J. 2020, 26, 2754-2760.
Systematic Synthesis of Tetrathia[8]circulenes: The Influence of Peripheral Substituents on the Structures and Properties in Solution and Solid States
S. Kato, S. Akahori, Y. Serizawa, X. Lin, M. Yamauchi, S. Yagai, T. Sakurai, W. Matsuda, S. Seki, H. Shinokubo, Y. Miyake
J. Org. Chem. 2020, 85, 62-69.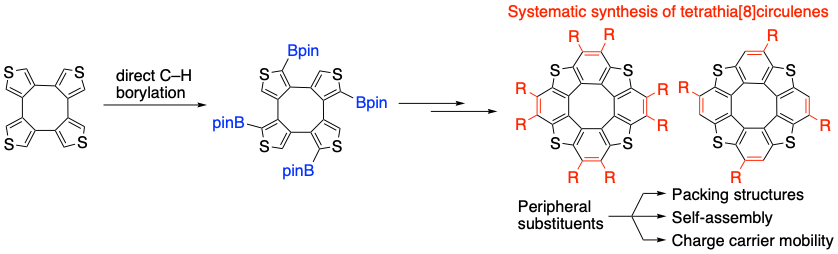
Soluble and Planar 2,9-Diazaperopyrenes through Reductive Aromatization of Perylene Diimides: Tunable Emission and Aggregation Behaviors
Y. Nakamura, T. Nakazato, T. Kamatsuka, H. Shinokubo, Y. Miyake
Chem. Eur. J. 2019, 25, 10571-10574.
Regioselective Oxidative Ring Cleavage of Antiaromatic Nickel(II) Norcorrole to Dialkoxybis(dipyrrin)s
S. A. Shafie, H. Kawashima, Y. Miyake, H. Shinokubo
ChemPlusChem 2019, 84, 623-626.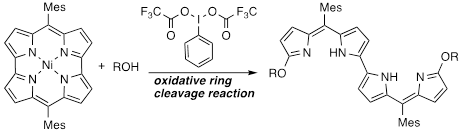
Diazachlorin and Diazabacteriochlorin for One- and Two-Photon Photodynamic Therapy
J. Longevial, A. Yamaji, D. Aggad, G. Kim, W. X. Chia, T. Nishimura, Y. Miyake, S. Clément, J. Oh, M. Daurat, C. Nguyen, D. Kim, M. Gary-Bobo, S. Richeter, H. Shinokubo
Chem. Commun. 2018, 54, 13829-13832.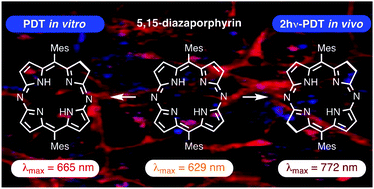
The Reductive Aromatization of Naphthalene Diimide: A Versatile Platform for 2,7-Diazapyrenes
T. Nakazato, T. Kamatsuka, J. Inoue, T. Sakurai, S. Seki, H. Shinokubo, Y. Miyake
Chem. Commun. 2018, 54, 5177-5180.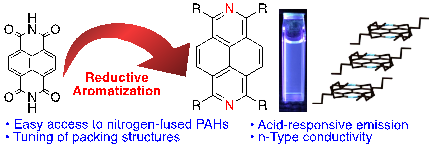
Synthesis and Photodynamics of Tetragermatetrathia[8]circulene
S. Akahori, H. Sakai, T. Hasobe, H. Shinokubo, Y. Miyake
Org. Lett. 2018, 20, 304-307.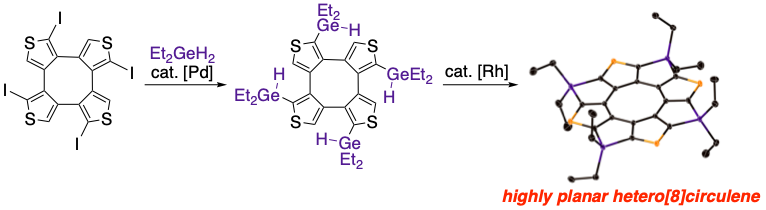
Structures of the Heme Acquisition Protein HasA with Iron(III)-5,15-Diphenyl-Porphyrin and Derivatives Thereof as an Artificial Prosthetic Group
H. Uehara, Y. Shisaka, T. Nishimura, H. Sugimoto, Y. Shiro, Y. Miyake, H. Shinokubo, Y. Watanabe, O. Shoji
Angew. Chem. Int. Ed. 2017, 56, 15279-15283.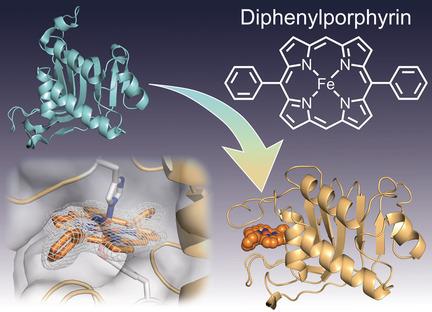
Design and Synthesis of Tunable Ligands with 4,4′-Bipyridyl as an Electron Accepting Unit and Their Rhenium Complexes
T. Kamatsuka, H. Shinokubo, Y. Miyake
Organometallics 2017, 36, 3429-3434.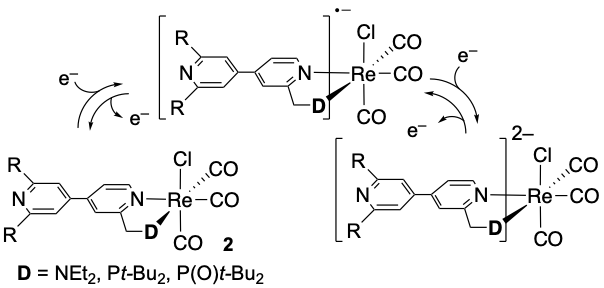
Selective α-Arylation of α,β-Unsaturated Carbonyl Compounds Mediated by a Visible Light Photoredox Catalyst
Y. Ando, T. Kamatsuka, H. Shinokubo, Y. Miyake
Chem. Commun. 2017, 53, 9136-9138.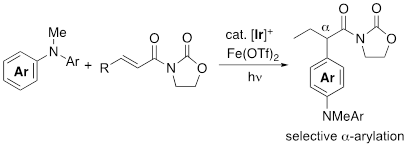
Synthesis of Tetraaza[8]circulenes from Tetrathia[8]circulenes through an SNAr-Based Process
Y. Nagata, S. Kato, Y. Miyake, H. Shinokubo
Org. Lett. 2017, 19, 2718-2721.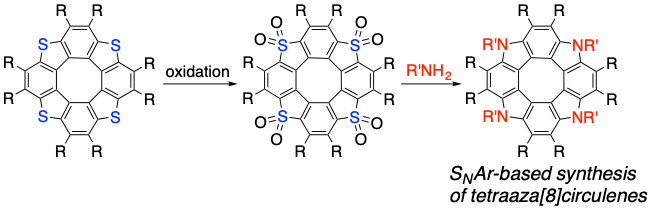
Synthesis, Properties and Reactivities of Ruthenium(II) Carbonyl 5,15-Diazaporphyrins
Tsubasa Nishimura, Yoshihiro Miyake,* Hiroshi Shinokubo
Chem. Lett. 2017, 46, 995-997.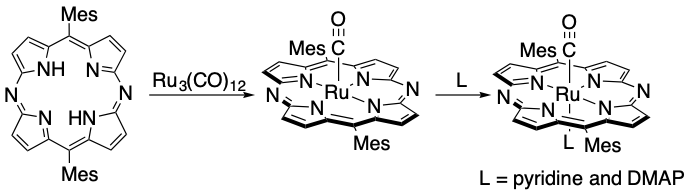
Synthesis of Tetrasilatetrathia[8]circulenes by a Fourfold Intramolecular Dehydrogenative Silylation of C–H Bonds
Y. Serizawa, S. Akahori, S. Kato, H. Sakai, T. Hasobe, Y. Miyake, Hiroshi Shinokubo
Chem. Eur. J. 2017, 23, 6948-6952.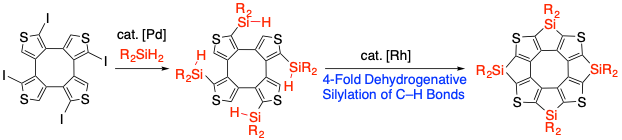
Chemo- and Regioselective Reduction of 5,15-Diazaporphyrins Providing Antiaromatic Azaporphyrinoids
A. Yamaji, H. Tsurugi, Y. Miyake, K. Mashima, H. Shinokubo
Chem. Eur. J. 2016, 22, 3956-3961.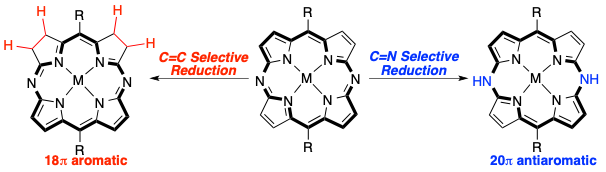
Diversity-Oriented Synthesis of Tetrathia[8]circulenes by Sequential C–H Borylation and Annulation
S. Kato, Y. Serizawa, D. Sakamaki, S. Seki, Y. Miyake, H. Shinokubo
Chem. Commun. 2015, 51, 16944-16947.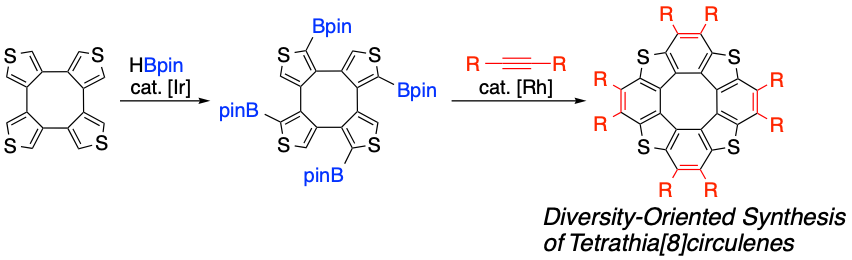
Copyright© 2024
Organic Chemistry Laboratory. All Right Reserved.
Produced by coanet